Contact us
Our team would love to hear from you.

Technology has made substantial strides in transforming healthcare—this is a widely recognized fact. Thanks to technological advancements, healthcare is increasingly adopting a user-centric approach, significantly improving service quality, and achieving much more in terms of efficiency, care, and diagnostics. However, with changes come challenges. One of the most crucial of these challenges is ensuring that technological advancements do not cause harm.
When it comes to medical device software, failure can lead to serious health implications or even loss of life, so it’s imperative that such software be safe and effective. A regulatory framework is necessary to prevent the emergence of low-quality software products and enforce rigorous testing at all stages of development.
The IEC 62304 standard establishes a framework for all the processes involved in the medical device software development lifecycle, focusing on risk management. It also defines the requirements for development and testing, with a particular emphasis on safety. Therefore, when building medical device software, it is imperative to establish and adhere to a thorough testing process that aligns with the stringent requirements of IEC 62304 and all associated standards. This commitment goes beyond mere adherence, emphasizing dedication to patient safety and the continuous improvement of healthcare technology.
In this article, we explore the specifics of medical device software testing and delve into the details of the required testing protocols and the impact they have on the development of medical device software.
In medical device software development, the establishment of a comprehensive test plan is more than just a procedural step—it’s a security measure that aligns with the critical nature of the products being developed. The IEC 62304 standard highlights the multifaceted role of the test plan, which serves as a foundation for the validation and verification process within the regulatory framework.
Although the standard does not explicitly mandate the test plan structure and content, a comprehensive version under IEC 62304 might include the following key components:
The complexity of the test plan is determined by the device’s safety classification. For Class A devices, the plan may be straightforward, whereas Class C devices require a more exhaustive approach to address the higher risk profile. It’s crucial that the test plan provides full coverage to ensure that all aspects of the software are thoroughly tested for safety and reliability.
Moreover, the plan should accommodate both manual and automated testing methods to combine thoroughness with efficiency. Automation plays a key role in handling repetitive, data-intensive tests, allowing for more efficient resource use and consistent execution.
It is worth noting that testing documentation is a confirmation of the thorough testing process. Properly designed and recorded test cases and protocols are critical to the plan. They must be clear, replicable, and directly tied to specific requirements to ensure that every function is tested against its intended performance.
QA expert at EffectiveSoft
Moreover, clear records of test execution, results, and any actions taken in response to findings must be maintained. This documentation is not only a regulatory requirement but also provides transparency and traceability throughout the software’s development life cycle.
Following a meticulously established testing plan, the second critical layer of the medical device software security strategy involves a series of well-defined testing types. According to IEC 62304, these include, unit, integration, system, performance, and safety testing, each of which plays a specific role in the quality assurance framework.
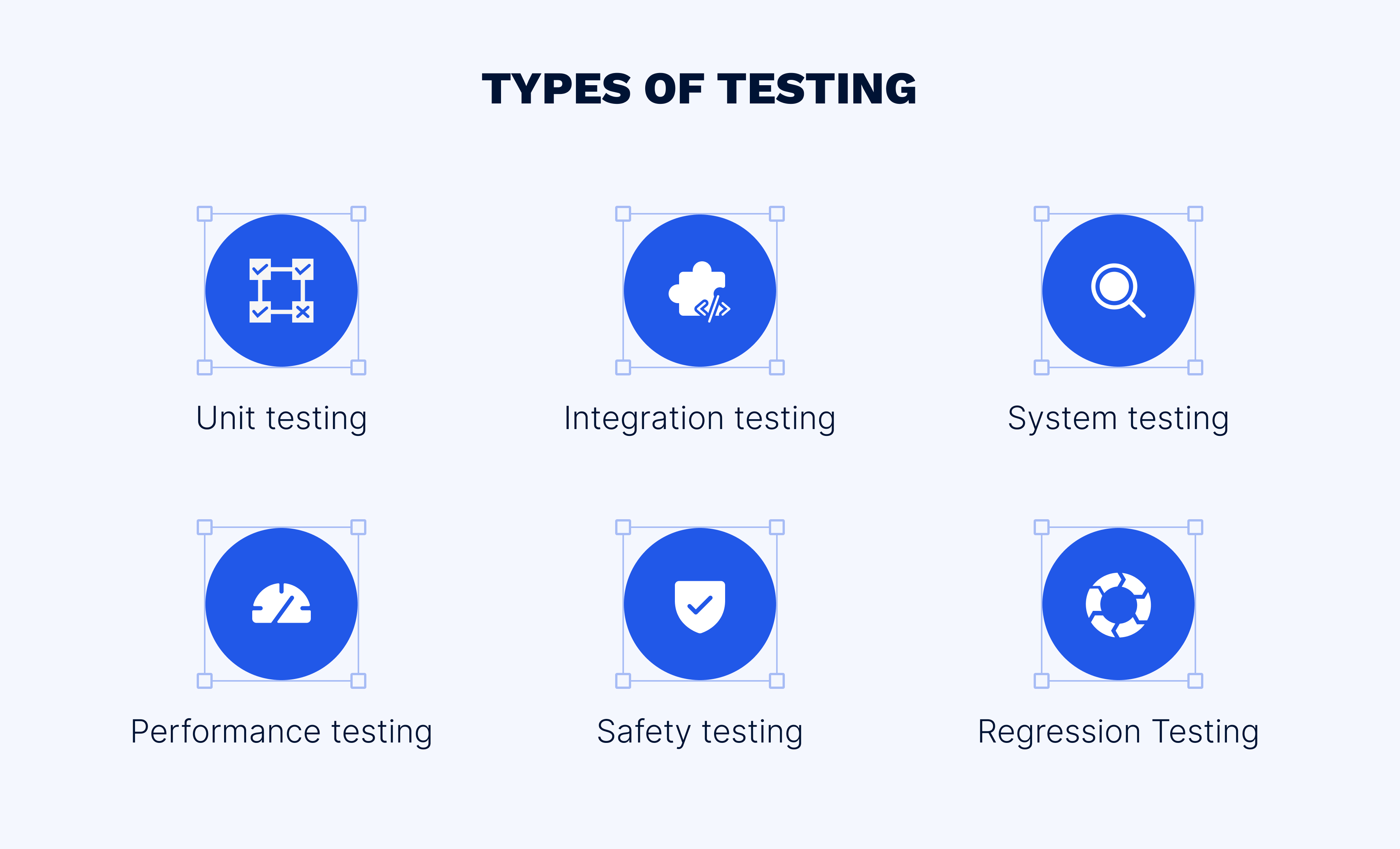
Unit testing involves testing individual units or components of the software in isolation. For medical devices, this can be a controller module or an algorithm class. Ensuring each unit performs as intended is critical in programs where the smallest failure might cause serious health implications.
Integration testing combines individual units and tests them as a group to expose faults in the interaction between integrated components. For example, in an automated insulin pump, integration testing would assess how the glucose monitoring module communicates with the insulin delivery mechanism, ensuring the entire system works cohesively.
System testing evaluates the complete software product to ensure it complies with the specified requirements. For an MRI machine, system testing would involve examining all software components to verify that they all function correctly, enabling accurate imaging and ensuring patient safety.
Performance testing assesses the responsiveness, stability, scalability, and resource usage of the software under a particular workload. This is crucial for medical devices like pacemakers, where the software must operate effectively under various patient’s conditions and respond correctly in emergency scenarios.
Safety testing is an exhaustive examination of the software’s ability to react to a range of error conditions. For instance, safety testing for a radiation therapy system would ensure that software failsafes activate appropriately to prevent a radiation overdose.
Each of these testing types is mandatory not only for compliance but also for patient safety and product reliability.
In the spectrum of testing types, regression testing plays a distinctive role, for all, in the validation of changes made to medical device software. In line with IEC 62304’s focus on risk management throughout the product life cycle, regression testing is an ongoing process that validates improvements of any kind, including new features, bug fixes, and performance improvements. Regression testing protects the existing components and functionalities from adverse impacts.For example, if a diagnostic device’s software is updated to improve its user interface, regression testing must be conducted to confirm that the update hasn’t affected the diagnostic algorithms or the device’s ability to communicate with other systems.
For example, if a diagnostic device’s software is updated to improve its user interface, regression testing must be conducted to confirm that the update hasn’t affected the diagnostic algorithms or the device’s ability to communicate with other systems.
To facilitate continuous, efficient regression testing, it is particularly important to integrate the implementation of test automation into the regression testing process.Test automation allows tests to be run quickly and consistently while ensuring that medical device software is rigorously examined after every change. Furthermore, the use of automated testing frameworks and tools significantly reduces the time required for regression testing. This enables frequent re-validation of the medical device software without compromising quality or compliance. For example, a blood analysis device’s firmware update would traditionally require a manual testing regimen that could take days to complete. With automation, the same regression tests could be executed overnight, highlighting any failures that the update may have caused, thereby accelerating the development cycle and ensuring continuous delivery of high-quality software.
The precision and strictness of unit, integration, system, performance, and safety testing, coupled with the thoroughness of regression testing, create a continuous validation process that affirms the stringent requirements outlined in the IEC 62304 standard.
To demonstrate that requirements established in the test plan are tested and documented at each development stage, a structured verification and validation (V&V) approach is necessary. V&V are critical components of the medical device software development lifecycle according to IEC 62304. To guide the V&V process, the IEC standard recommends following the V-model. The V-model offers a clear methodology for aligning development activities with the necessary verification steps to validate requirements and testing goals. This section will explain the V-model approach and clarify the distinctions between verification and validation according to IEC 62304.
The V-model is a visual representation of the software development process, with the left side detailing the development phases and the right side focused on the corresponding testing activities. The model emphasizes a step-by-step approach, in which the output of each phase is thoroughly verified before moving on to the next stage.
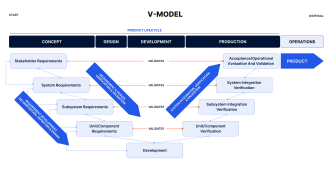

User Requirements Specifications (URS): Starting point covering user needs.
System Requirements Specifications (SRS): Technical specifications derived from user needs.
Software Architecture Design: High-level design outlining the structure of the software.
Software Detailed Design: Creation of detailed software component designs.
Unit Tests: Verification of individual software components against the respective phase output and requirements.
Integration Tests: Verification of the interaction between components.
System Tests: Verification against system requirements.
Acceptance Testing: Ensuring the software meets URS and is suitable for use.
Software Validation: The culmination of the V-model, validating that the overall software meets all user needs and operational requirements in the intended environment.
Verification, in the context of IEC 62304 and the V-model, ensures that each step of the software development process is executed correctly. It involves activities such as code reviews, unit tests, integration tests, and static and dynamic analysis. Verification ensures that the medical device software meets its specified requirements at every stage of development.
Validation, on the other hand, focuses on whether the final product fulfills its intended use when placed in its intended environment. It involves activities such as clinical trials, user acceptance testing, and final software validation. Validation confirms that the software meets the needs and requirements of end users.
IEC 62304 mandates V&V throughout the software development lifecycle, requiring documented evidence that the software has been developed and tested according to its intended use and corresponding safety requirements.
While the V-model supports IEC 62304 by providing a clear framework for aligning development activities with necessary V&V steps, traceability and coverage metrics embedded in this process demonstrate software conformance to standards, maintaining the integrity of medical device software within the healthcare industry.
In medical device software development, every requirement must be addressed through corresponding test cases. Furthermore, integrating IEC 62304 with ISO 14971 for risk management and ISO 13485 for quality management systems is crucial to ensure continuous compliance and medical device software reliability.
To achieve maximum test case coverage, follow these best practices:
A traceability matrix is an essential tool for tracking the coverage of each requirement through the various stages of software development and testing. It is a comprehensive document that connects requirements to design elements, test cases, and test results.
Features of an effective traceability matrix:
While IEC 62304 sets the requirements for the lifecycle of medical device software, concentrating on development and maintenance processes, ISO 14971 complements this by providing a framework for risk management throughout the product life cycle. Together, they create a comprehensive approach to safety and risk management. The integration of these standards requires:
In addition to the above, ISO 13485 outlines quality management system requirements. It plays a pivotal role in test process management by ensuring:
Implementing test cases in medical device software development is fraught with challenges. These can range from evolving requirements and regulatory changes to technological advancements and resource constraints. To overcome challenges, the following best practices can be implemented:
Involving the QA team from the start of the development process helps in understanding the requirements and planning effective test strategies.
Prioritizing test cases based on risk assessment ensures that the most critical functionalities are tested thoroughly.
Implementing CI/CD practices assists in the early detection of defects and reduces the time to market.
Adequate training ensures that the team is well-versed with the relevant standards and equipped with the latest knowledge and tools to perform testing effectively.
Implementing best practices significantly helps establish a robust testing framework that can adapt to changes and maintain the highest quality standards in the face of any challenge.
QA expert at EffectiveSoft
Effective testing of medical device software, as guided by the IEC 62304 standard, is indispensable for the delivery of safe and reliable healthcare technologies. Employing a structured approach such as the V-model, alongside thorough test planning and traceability, is key to robust validation efforts. As medical devices grow more complex, manufacturers and healthcare developers must embrace continuous improvement and innovation in testing practices to uphold the highest safety and performance standards. Adherence to these strict processes not only builds confidence among users but also drives the forward momentum of medical technology, enhancing patient care. At EffectiveSoft, we have a team of QA engineers with considerable experience in medical device software testing. Contact us to discuss you project.
Our team would love to hear from you.
Fill out the form, and we’ve got you covered.
What happens next?
San Diego, California
4445 Eastgate Mall, Suite 200
92121, 1-800-288-9659
San Francisco, California
50 California St #1500
94111, 1-800-288-9659
Pittsburgh, Pennsylvania
One Oxford Centre, 500 Grant St Suite 2900
15219, 1-800-288-9659
Durham, North Carolina
RTP Meridian, 2530 Meridian Pkwy Suite 300
27713, 1-800-288-9659
San Jose, Costa Rica
C. 118B, Trejos Montealegre
10203, 1-800-288-9659