
Contact us
Our team would love to hear from you.
A single secure environment for collaboration of medical specialists during clinical trials of new drugs and medical devices.

Sentral Clinical Research Services, LLC (SCRS) is a U.S. company specializing in medical research of new drugs and medical devices. The company’s aim is to facilitate the participation of physicians and patients in clinical research.
A clinical trial is a complex process that involves a lot of documentation and cooperation of many medical specialists both at dedicated research facilities and in the private practice setting. Physicians’ collaboration and medical data analysis constitute an important part of the process.
As the amount of research data expanded, the company reached out to EffectiveSoft to develop a unified document management system that would be highly flexible and user-friendly, keeping document version control and approval workflows.
Sentral Clinical Research Services, LLC
USA
Custom software development
.NET, C#, Angular JS, MsSQL
The customer had only a general idea of what the system should look like. During the regular meetings our team discussed the system wireframes and functional specifications with the client to ensure that all the customer’s requirements were met.
As a result, our engineers developed the document management system SentralBinders. It simplifies teamwork, expedites document exchange (in Microsoft Word and Adobe PDF formats), and ensures mobility of participants due to access available from different devices (both PCs and iPads).

Transparent and automated workflow
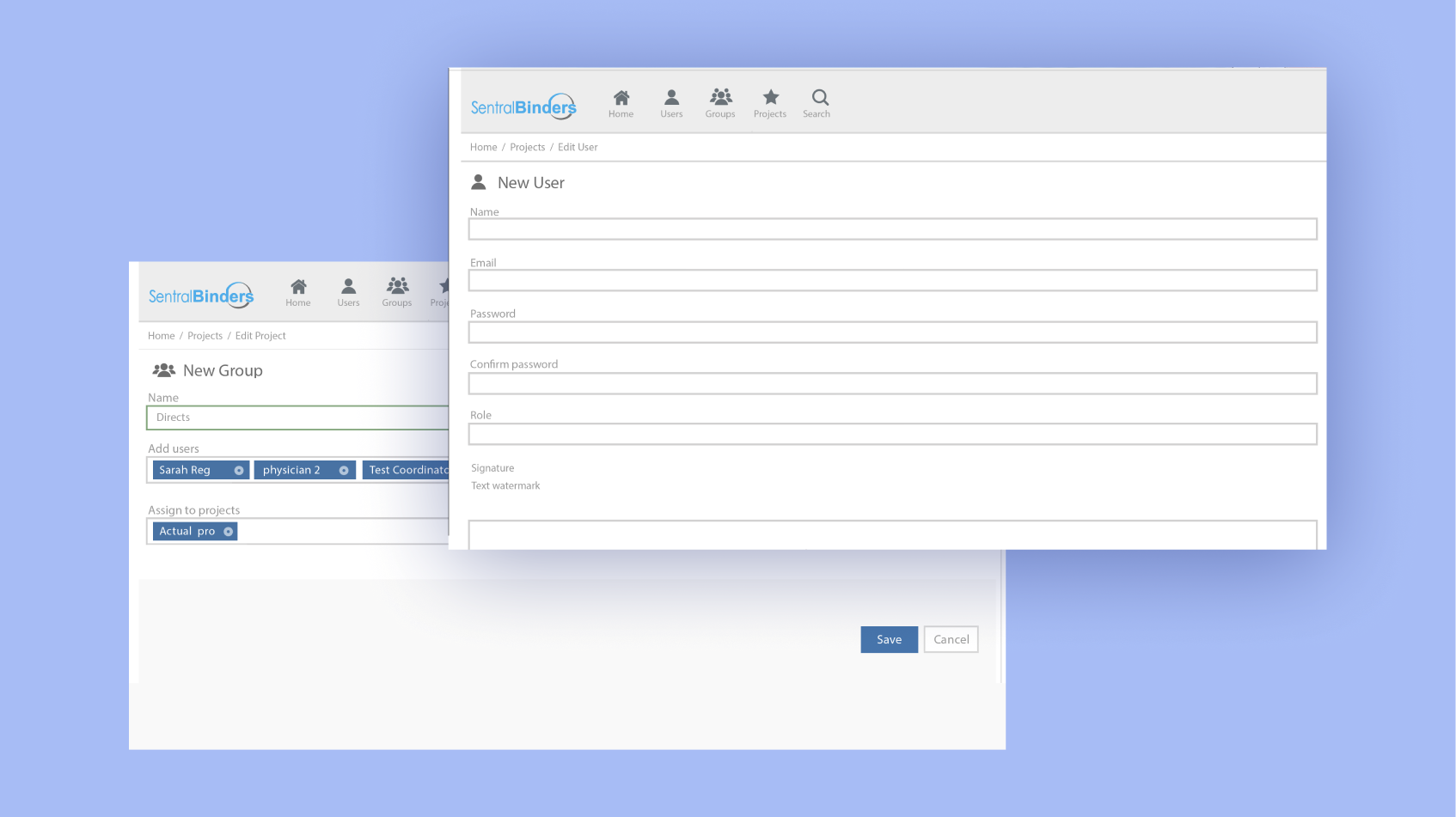
Flexible and reliable data management
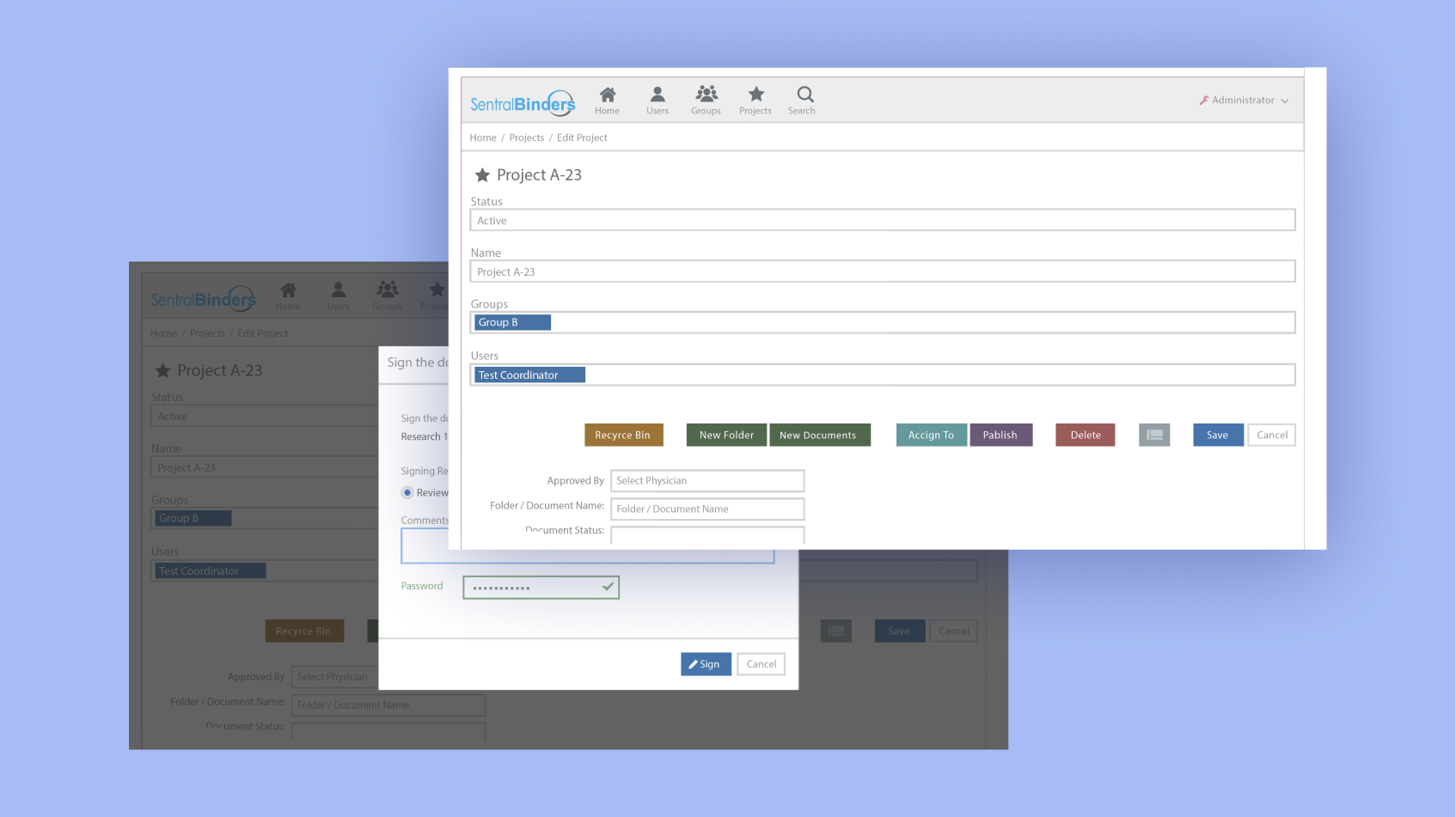
Simplified version control and history tracking
Our team would love to hear from you.
Fill out the form, and we’ve got you covered.
What happens next?
San Diego, California
4445 Eastgate Mall, Suite 200
92121, 1-800-288-9659
San Francisco, California
50 California St #1500
94111, 1-800-288-9659
Pittsburgh, Pennsylvania
One Oxford Centre, 500 Grant St Suite 2900
15219, 1-800-288-9659
Durham, North Carolina
RTP Meridian, 2530 Meridian Pkwy Suite 300
27713, 1-800-288-9659
San Jose, Costa Rica
C. 118B, Trejos Montealegre
10203, 1-800-288-9659